Nuclear hazards
Radionuclides are elements (uranium 235, uranium 283, thorium 232, potassium 40, radium 226, carbon 14 etc.) with unstable atomic nuclei and on decomposition release ionizing radiations in the form of alpha, beta and gamma rays.
Out of the known 450 radioisotopes only some are of environmental concern like strontium 90, tritium, plutonium 239, argon 41, cobalt 60, cesium 137, iodine 131, krypton 85 etc. These can be both beneficial and harmful, depending on the way in which they are used.
We routinely use X-rays to examine bones for fractures, treat cancer with radiation and diagnose diseases with the help of radioactive isotopes. About 17% of the electrical energy generated in the world comes from nuclear power plants.
Radioactive substances when released into the environment are either dispersed or become concentrated in living organisms through the food chain. Other than naturally occurring radioisotopes, significant amounts are generated by human activity, including the operation of nuclear power plants, the manufacture of nuclear weapons, and atomic bomb testing.
For example, strontium 90 behaves like calcium and is easily deposited and replaces calcium in the bone tissues. It could be passed to human beings through ingestion of strontium-contaminated milk. Again another example is tritium, which is radioactive hydrogen.
The amount of tritium released from nuclear power plants to the atmosphere have reached as high as tens of thousands of curies in one year, and releases to bodies of water have measured as high as tens of millions of picocuries per litre. The U.S. Environmental Protection Agency standard for permissible levels of tritium in drinking water is 20,000 picocuries per litre.
Nuclear power plants routinely and accidentally release tritium into the air and water. Tritium has a half-life of 12.3 years and emits radioactive beta particles. Once tritium is inhaled or swallowed, its beta particles can bombard cells causing a mutation.
Few occupations that involve radioactive exposures are uranium mineworkers, radium watch dial painters, technical staff at nuclear power plants, etc. Exposure to radioactive and nuclear hazards has been clinically proven to cause cancer, mutations and teratogenesis (Teratogenesis is a prenatal toxicity characterized by structural or functional defects in the developing embryo or fetus).
Nuclear hazard effects can be either initial or residual. Initial effects occur in the immediate area of explosion and are hazardous immediately after the explosion where as the residual effects can last for days or years and cause death. The principal initial effects are blast and radiation.
Blast causes damage to lungs, ruptures eardrums, collapses structures and causes immediate death or injury. Thermal Radiation is the heat and light radiation, which a nuclear explosion’s fireball emits producing extensive fires, skin burns, and flash blindness. Nuclear radiation consists of intense gamma rays and neutrons produced during the first minute after the explosion.
This radiation causes extensive damage to cells throughout the body. Radiation damage may cause headaches, nausea, vomiting, diarrhea, and even death, depending on the radiation dose received.
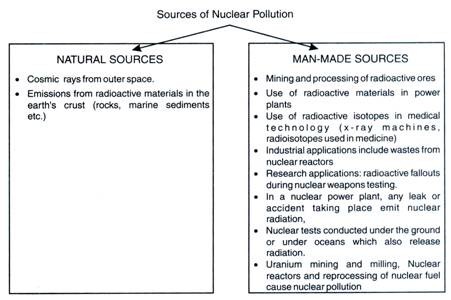
Sources of Nuclear Pollution:
The sources of radioactivity include both natural and manmade.
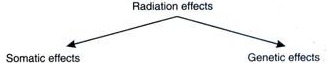
Effects of Nuclear Pollution:
Studies have shown that the health effects due to radiation are dependent on the level of dose, kind of radiation, duration of exposure and types of cells irradiated. Radiation effects can be somatic or genetic.
1. Somatic effects:
Somatic effects the function of cells and organs. It causes damages to cell membranes, mitochondria and cell nuclei resulting in abnormal cell functions, cell division, growth and death.
2. Genetic effects:
Genetic effects the future generations. Radiations can cause mutations, which are changes in genetic makeup of cells. These effects are mainly due to the damages to DNA molecules. People suffer from blood cancer and bone cancer if exposed to doses around 100 to 1000 roentgens. Instantaneous deaths on exposure in the event if disasters are many.
Management of Radioactive Waste:
a. The radioactive waste which comes out from industry, nuclear reactors should be stored and allowed to decay either naturally in closed drums or in very large underground air tight cemented tanks (Delay and Decay).
b. The intermediate radioactive waste should be disposed off into the environment after diluting it with some inert materials (Dilute and Disperse)
c. Now-a-days small quantities of high activity wastes are converted into solids such as concrete and then it is buried underground or sea. (Concentrate and contain)
Control Measures:
a. Laboratory generated nuclear wastes should be disposed off safely and scientifically.
b. Nuclear power plants should be located in areas after careful study of the geology of the area, tectonic activity and meeting other established conditions.
c. Appropriate protection against occupational exposure.
d. Leakage of radioactive elements from nuclear reactors, careless use of radioactive elements as fuel and careless handling of radioactive isotopes must be prevented.
e. Safety measure against accidental release of radioactive elements must be ensured in nuclear plants.
f. Unless absolutely necessary, one should not frequently go for diagnosis by x-rays.
g. Regular monitoring of the presence of radioactive substance in high risk area should be ensured.
Among the many options for waste disposal, the scientists prefer to bury the waste in hundreds of meters deep in the earth’s crust is considered to be the best safety long term option.