Copolymerization
When two or more different monomers unite together to polymerize, their result is called a copolymer and its process is called copolymerization.
Commercially relevant copolymers include acrylonitrile butadiene styrene(ABS), styrene/butadiene co-polymer(SBR), nitrile rubber, styrene-acrylonitrile, styrene-isoprene-styrene (SIS) and ethylene-vinyl acetate.
Types of copolymers
Different types of copolymers
Since a copolymer consists of at least two types of constituent units (also structural units), copolymers can be classified based on how these units are arranged along the chain.These include:
- Alternating copolymers with regular alternating A and B units (2)
- Periodic copolymers with A and B units arranged in a repeating sequence (e.g. (A-B-A-B-B-A-A-A-A-B-B-B)n)
- Statistical copolymers are copolymers in which the sequence of monomer residues follows a statistical rule. If the probability of finding a given type monomer residue at a particular point in the chain is equal to the mole fraction of that monomer residue in the chain, then the polymer may be referred to as a truly random copolymer(3).
- Block copolymers comprise two or more homopolymer subunits linked by covalent bonds (4). The union of the homopolymer subunits may require an intermediate non-repeating subunit, known as a junction block. Block copolymers with two or three distinct blocks are called diblock copolymers and triblock copolymers, respectively.
Copolymers may also be described in terms of the existence of or arrangement of branches in the polymer structure.Linear copolymers consist of a single main chain whereas branched copolymers consist of a single main chain with one or more polymeric side chains.
Other special types of branched copolymers include star copolymers, brush copolymers, and comb copolymers. Ingradient copolymers the monomer composition changes gradually along the chain.
A terpolymer is a copolymer consisting of three distinct monomers. The term is derived from ter (Latin), meaning thrice, and polymer.
- Stereoblock copolymers
A special structure can be formed from one monomer where now the distinguishing feature is the tacticity of each block.
Graft copolymers
Graft copolymers are a special type of branched copolymer in which the side chains are structurally distinct from the main chain. The illustration (5) depicts a special case where the main chain and side chains are composed of distinct homopolymers. However, the individual chains of a graft copolymer may be homopolymers or copolymers. Note that different copolymer sequencing is sufficient to define a structural difference, thus an A-B diblock copolymer with A-B alternating copolymer side chains is properly called a graft copolymer.
For example, suppose we perform a free-radical polymerization of styrene in the presence of polybutadiene, a synthetic rubber, which retains one reactive C=C double bond per residue. We get polystyrene chains growing out in either direction from some of the places where there were double bonds, with a one-carbon rearrangement. Or to look at it the other way around, the result is a polystyrene backbone with polybutadiene chains growing out of it in both directions. This is an interesting copolymer variant in that one of the ingredients was a polymer to begin with.
As with block copolymers, the quasi-composite product has properties of both “components”. In the example cited, the rubbery chains absorb energy when the substance is hit, so it is much less brittle than ordinary polystyrene. The product is called high-impact polystyrene, or HIPS.
Block copolymers
A special kind of copolymer is called a “block copolymer”. Block copolymers are made up of blocks of different polymerizedmonomers. For example, PS-b-PMMA is short for polystyrene-b-poly(methyl methacrylate) and is usually made by first polymerizing styrene, and then subsequently polymerizing MMA from the reactive end of the polystyrene chains. This polymer is a “diblock copolymer” because it contains two different chemical blocks. Triblocks, tetrablocks, multiblocks, etc. can also be made. Diblock copolymers are made using living polymerization techniques, such as atom transfer free radical polymerization (ATRP), reversible addition fragmentation chain transfer (RAFT), ring-opening metathesis polymerization(ROMP), and living cationic or living anionic polymerizations. An emerging technique is chain shuttling polymerization. The most powerful strategy to prepare block copolymers is the chemoselective stepwise coupling between polymeric precursors and heterofunctional linking agents.This method enables access to peculiarly exotic structures such as tetrablock quarterpolymers ABCD.
Recent research in block copolymers suggests that they may be useful in creating self-constructing fabrics with potential utility in semiconductor arrays (for example, computer memory devices) by assembling fine details atop a structured base created using conventional microlithography methods.
Phase separation
SBS block copolymer in TEM
Block copolymers are interesting because they can “microphase separate” to form periodic nanostructures, as in the styrene-butadiene-styrene block copolymer shown at right. The polymer is known as Kraton and is used for shoe soles and adhesives. Owing to the microfine structure, the transmission electron microscope or TEM was needed to examine the structure. The butadiene matrix was stained with osmium tetroxide to provide contrast in the image. The material was made by living polymerization so that the blocks are almost monodisperse, so helping to create a very regular microstructure. The molecular weight of the polystyrene blocks in the main picture is 102,000; the inset picture has a molecular weight of 91,000, producing slightly smaller domains.
SBS block copolymer schematic microstructure
Microphase separation is a situation similar to that of oil and water. Oil and water are immiscible – they phase separate. Due to incompatibility between the blocks, block copolymers undergo a similar phase separation. Because the blocks are covalently bonded to each other, they cannot demix macroscopically as water and oil. In “microphase separation” the blocks form nanometer-sized structures. Depending on the relative lengths of each block, several morphologies can be obtained. In diblock copolymers, sufficiently different block lengths lead to nanometer-sized spheres of one block in a matrix of the second (for example PMMA in polystyrene). Using less different block lengths, a “hexagonally packed cylinder” geometry can be obtained. Blocks of similar length form layers (often called lamellae in the technical literature). Between the cylindrical and lamellar phase is the gyroid phase. The nanoscale structures created from block copolymers could potentially be used for creating devices for use in computer memory, nanoscale-templating and nanoscale separations.
Polymer scientists use thermodynamics to describe how the different blocks interact. The product of the degree of polymerization, n, and the Flory-Huggins interaction parameter,  , gives an indication of how incompatible the two blocks are and whether or not they will microphase separate. For example, a diblock copolymer of symmetric composition will microphase separate if the product
, gives an indication of how incompatible the two blocks are and whether or not they will microphase separate. For example, a diblock copolymer of symmetric composition will microphase separate if the product 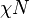 is greater than 10.5. If
is greater than 10.5. If 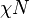 is less than 10.5, the blocks will mix and microphase separation is not observed. The incompatibility between the blocks also affects the solution behavior of these copolymers and their adsorption behavior on various surfaces
is less than 10.5, the blocks will mix and microphase separation is not observed. The incompatibility between the blocks also affects the solution behavior of these copolymers and their adsorption behavior on various surfaces

