Nitrogen cycle
Nitrogen Cycle: Useful notes on Nitrogen Cycle!
Nitrogen is the most prevalent element in living organisms. Nitrogen is a constituent of amino acids, proteins, hormones, chlorophylls and many of the vitamins. Plants compete with microbes for the limited nitrogen that is available in soil. Nitrogen exists as two nitrogen atoms joined by a very strong triple covalent bond (N a”? N).
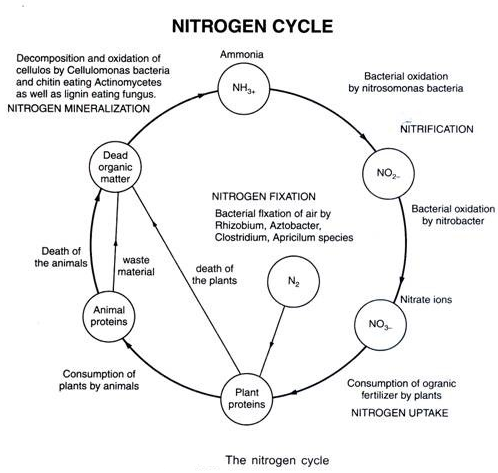
The process of conversion of nitrogen (N2) to ammonia is termed as nitrogen fixation. In nature, lightning and ultraviolet radiation provide enough energy to convert nitrogen to nitrogen oxides (NO, NO2, N2O). Industrial combustions, forest fires, automobile exhausts and power-generating stations are also sources of atmospheric nitrogen oxides. Decomposition of organic nitrogen of dead plants and animals into ammonia is called ammonification.
Ammonia is first oxidized to nitrite by the bacteria Nitrosamines and/or Micrococcus. The nitrite is further oxidized to nitrate with the help of the bacterium Nitrobacteria. These steps are called nitrification. These nitrifying bacteria are chemoautotrophs.
The nitrate thus formed is absorbed by plants and is transported to the leaves. In leaves, it is reduced to form ammonia that finally forms the amine group of amino acids. Nitrate present in the soil is also reduced to nitrogen by the process of densification.
Plants absorb a wide variety of mineral elements. Not all the mineral elements that they absorb are required by plants. Out of the more than 105 elements discovered so far, less than 21 are essential and beneficial for normal plant growth and development. The elements required in large quantities are called macronutrients while those required in less quantities or in trace are termed as micronutrients.
These elements are either essential constituent of proteins, carbohydrates, fats, nucleic acid etc., and/or take part in various metabolic processes. Deficiency of each of these essential elements may lead to symptoms called deficiency symptoms. Chlorosis, necrosis, stunted growth, impaired cell division, etc., are some prominent deficiency symptoms. Plants absorb minerals through roots by either passive or active processes.
Plants cannot use atmospheric nitrogen directly. But some of the plants in association with N2-fixing bacteria, especially roots of legumes, can fix this atmospheric nitrogen into biologically usable forms. Nitrogen fixation requires a strong reducing agent and energy in the form of ATP. N2-fixation is accomplished with the help of nitrogen-fixing microbes, mainly Rhizobium. The enzyme nitrogenous which plays an important role in biological N2fixation is very sensitive to oxygen. Most of the processes take place in anaerobic environment. The energy, ATP, required is provided by the respiration of the host cells.