Air pollution
We have seen that the main sources of air pollution are:
(i) Motor vehicles,
(ii) Industries, particularly their chimney wastes,
(iii) Fossil-fuel (coal) based plants, as thermal power plants.
Steps are to be taken to control pollution at source (prevention) as well as after the release of pollutants in the atmosphere. There is an urgent need to prevent the emissions from the above said major sources of air pollution.
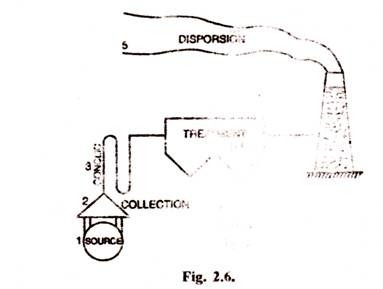
[Five points of control of possible emission of air pollutants]The control of emissions can be realized in number of ways.
Five separate possibilities for control are shown in Fig. 2.6.
These are briefly considered here as follows:
1. Source correction:
1 his is the easiest solution to air pollution problem, where we stop the guilty process. Hence, it is also called prevention. The engineer must consider the possibility of controlling the emissions by changing the process. For instance, if automobiles are found to release high lead levels in air, the most reasonable solution is simply to eliminate the lead in the gasoline. The source has been corrected and the problem solved.
In addition to a change of raw material, a modification of the process might also be used to achieve a desired result. For example municipal refuse incinerators are known to stink. The odours can often be readily controlled if the incinerators are operated at a high enough temperature to completely oxidise the organics which cause the odour. Such measures as process change, raw material conversion or equipment modification to meet emission standards are known as controls.
In contrast, abatement is the term used for all devices and methods for decreasing the quantity of pollutants leaching the atmosphere once the stuff has already been emitted from the source. In a wider sense and for simplicity, it is better to refer to all of the procedures as controls.
2. Collection of pollutants:
Often the most serious problem in air pollution control is the collection of the pollutants so as to provide treatment. Automobiles are most dangerous, the only because the emissions cannot be readily collected. If we could cannel exhausts from automobiles to some central facilities, their treatment would be much more reasonable than controlling each individual car.
One success in collecting pollutants has been the recycling of blowy gases in the internal combustion engine. By reigniting these gases and emitting them through the car’s exhaust system, the need of installing a separate treatment device for the car can be eliminated. Air pollution control engineers have their toughest time when the pollutants from an industry arc not collected but emitted from windows, doors etc.
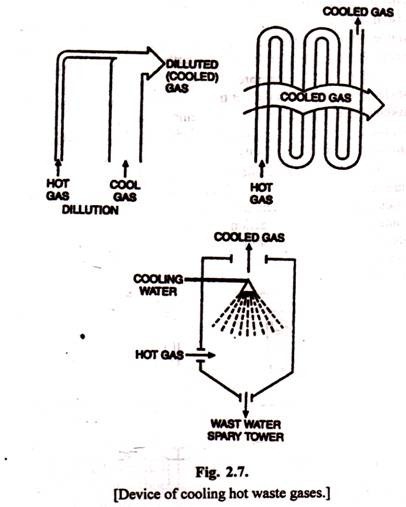
3. Cooling:
The exhaust gases to be treated are sometimes too hot for the control equipment and the gases must first be cooled. This can be done in three general ways: dilution, quenching, or heat exchange coils (Fig. 2.7). Dilution is acceptable only if the total amount of hot exhaust is small. Quenching has the additional advantage of scrubbing out some of these gases and particulates. The cooling coils are perhaps the most widely used, and are especially appropriate when heat can be conserved.
4. Treatment:
The selection of the correct treatment device requires the matching of the characteristics of the pollutant and the features of the control device. It is important to realise that the sizes of air pollutants range many orders of magnitude, and it is therefore not reasonable to expect one device to be effective for all pollutants.
In addition, the types of chemicals in emissions will often dictate the use of some devices. For example, a gas containing a high concentration of S02 could be cleaned by water sprays, but the resulting H2SO4 might present serious corrosion problems.
Many devices appear in the market, the following are the most widely used:
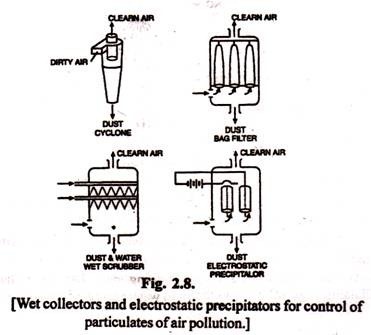
(a) Selling chambers are nothing more than large places in the flues, similar to settling tanks in water treatment. These chambers remove only the large particulates.
(b) Cyclones are widely used for removing large particulates. The dirty air is blasted into a conical cylinder, but off the centerline. This creates a violent swirl within the cone, and the heavy solids migrate to the wall of the cylinder where they slow down due to friction and exist at the bottom of the cone. The clean air is in the middle of the cylinder and exits out the top. Cyclones are widely used as pre-cleaners, to remove the heavy material before further treatment.
(c) Bag filters operate like the common vacuum cleaners. Fabric bags are used to collect the dust which must be periodically shaken out of the bags. The fabric removes nearly all particulates. Bag filters are widely used in many industries; but are sensitive to high temperature and humidity.
(d) Wet collectors come in many shapes and styles. The simple spray tower (Fig. 2.8) is an effective method for removing large particulates. More efficient scrubbers promote the contact between air and water by violent action in a narrow section into which the water is introduced. Generally, the more violent the encounter, and hence the smaller the gas bubbles or water droplets, the more effective the scrubbing.
(e) Electrostatic precipitators are widely used in power plants. The particulate matter is removed by first being charged by electrons (Jumping from one high voltage electrode to the other, and then migrating to the positively charged electrode. One type as shown in Fig. 2.8 consists of a pipe with a wire hanging down the middle. The particulates will collect on the pipe and must be removed by banging the pipes with hammers. Electrostatic precipitators have no moving parts, require electricity, and are extremely effective in removing sub micron particulates. They are expensive.
 (f) Gas scrubbers are simply wet collectors as described above but are used for dissolving the gases.
(f) Gas scrubbers are simply wet collectors as described above but are used for dissolving the gases.
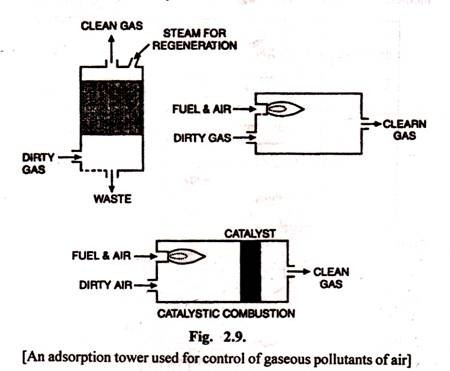
(g) Adsorption is the use of a material such as activated carbon to capture pollutants. Such adsorbers may be expensive to regenerate. Most of these work well for organics and have limited use for inorganic pollutants. Figure 2.9 shows the steps of an adsorption tower.
(h) Incineration is a method for removing gaseous pollutants by burning them to C02, H20 and inserts. This works only for combustible vapours.
(i) Catalytic combustion involves the use of a catalyst to adsorb or chemically change the pollutants.
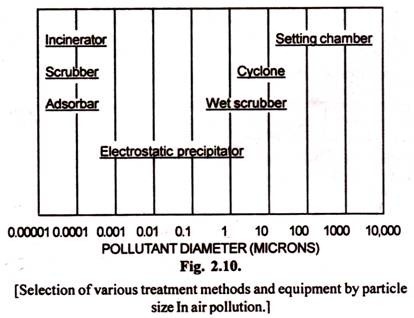
It is again important to emphasize the dependence of effectiveness of a treatment device on particle size. Fig. 2.10 shows the approximate ranges of the adaptability for the various treatment methods discussed above.
5. Dispersion:
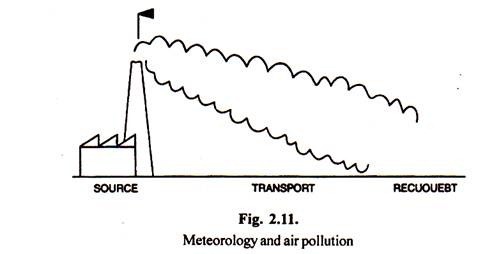
The science of meteorology has great bearing on air pollution. An air pollution problem involves three parts. The source, the movement of the pollutant, and the recipient (Fig. 2.10). The concentration of the pollutants at the recipient is affected by atmospheric dispersion, or how the pollutant is diluted with clean air. This dispersion takes place horizontally as well as vertically.
Earth rotation presents new areas for the sun to shine upon and to warm air. Accordingly a pattern of winds is set up around the world, some seasonal (e.g. hurricanes) and some permanent. Air pollution engineers often use a variation of the wind rose (a wind rose are graphic pictures of wind velocity and direction data), called a pollution rose to determine the source of a pollutant.
Diffusion is the process of spreading out the emission over a large area and thus reducing the concentration of the specific pollutants. The plume spread or dispersion is horizontal as well as vertical. The maximum concentration of pollutants is in the plume centre line, i.e. in the direction of the prevailing wind.
As we move further from the centre line, the concentration becomes lower. If we assume that the spread of a plume in both directions is approximated by a Gaussian probability curve, we can calculate the concentration of a pollutant at any distance X downwind from the source.
Pollution hazards can be predicted on the basis of meteorological data, and early warning for impending hazard conditions and emergency plans may be developed to close down industries.
Control of pollutants from moving sources:
Though many of the above said control methods can also apply to moving sources as well, one very special moving source the automobile-deserves special mention. Engine operation has direct effect on the emissions. Amount of CO, HC and NOx differs during idling, accelerating, cruising and decelerating.
Emission control techniques for the internal combustion automobile engine include tune-ups, catalytic reactors, and engine modifications. A tune-up may have a significant effect on emission components. For example, a high air/fuel ratio (a lean mixture) will reduce both CO and HC, but with increased NOx.
The second control strategy, now extensively used, is the catalytic reactor which oxidises the CO and HC to CO2 and H2 O. The second reactor reduces NOx to N2. The most popular catalyst reactors have two serious drawbacks. First, they are easily fouled by lead. Infact the move to non-lead gasoline has been prompted by this reason and not by the concern for lead levels in the atmosphere. The second problem with the reactors is that the sulphur compounds in gasoline are oxidised to particulate SO3, and thus increases the sulphur levels in environment.
In third control technique-engine modification, the stratified charge engine is used without catalytic reactions. In these engines the cylinders have two compartments, with one compartment receiving a rich mixture, igniting, and then providing a broad flame for an efficient bum in the main cylinder compartment. Other modifications have also been developed. It is difficult to manufacture a totally clean internal combusion engine. Electric cars are clean but they can store only limited power and thus their range is limited.
General methods to control air pollutions by automobiles and industries have been considered briefly above. Some specific measures to control vehicular and industrial pollutants in air are given below.
Vehicular pollution:
1. To check pollutant emission from vehicular exhaust:
This can be achieved by:
(i) Using new proportion of gasoline and air,
(ii) More exact timing of fuel feeding,
(iii) Using gas additives to improve combustion,
(iv) By injecting air into the exhaust to convert exhaust compounds to less toxic materials, and by
(v) Updating of engine design and/or install abatement equipment (device) to improve combustion with the existing engine design.
Carbon monoxide results from low air content of the fuel mixture, whereas NOx production is promoted by high combustion temperatures. Hydrocarbons follow more or less the pattern of CO.
The complete elimination of these three pollutants may be achieved by either updating the present design of engines (e.g. four stroke engines) or by making appropriate changes in devices for improving combustion.
2. To control evaporation from fuel tank and carburettor:
This can be done by:
(i) Collection of vapours with activated charcoal when engine is turned off and its ignition when the engine is started,
(ii) Subjecting gasoline in the tank to slight pressure to prevent the gas from evaporation and
(iii) Developing low-volatile gasoline that does not evaporate easily.
3. Use of filters:
Some gas vapours escape between walls and the piston which enters the crankcase and then discharge into atmosphere. Hydrocarbons (about 25%) are released in this way. Thus use of filters that capture and recycle these escaped gases in the engine should control the emission of these hydrocarbons.
4. Control through Law:
These are to be enforced some standards through Motor Vehicles Act and other Acts for design of engines etc.
Industrial pollution:
To check air pollution by industrial plant chimney wastes, we must devise measures for removal of the particulate matter and gaseous pollutants from the wastes. Removal of particulate matter involves their collection under the influence of different forces, thereby moving them continuously out of the gas stream.
The equipment used for their removal are:
(i) Cyclone collectors, and
(ii) Electrostatic precipitators (ESPs). Thus we have to generate the control technology. At present there are few power plants and industries that have installed the requisite ESPs.
1. Cyclone collectors:
Here the waste gas containing particles is subjected to centrifugation. The suspended particles move towards the wall of cyclone body, and then to its bottom and finally discharged out. The cyclone collectors remove about 70% of the particles.
2. Electrostatic precipitators (ESPs):
To remove the particles from gas stream, the electrical forces are applied within the chamber in the precipitator. Suspended particles become charged or ionised, and they are attracted to charged electrodes and then removed. ESPs can remove 99% of the particulate pollutants from chimney exhaust
ESPs work very well in power plants, paper mills, cement mills, carbon block plants etc. High resistivity dust may make separation in a ESP difficult. To overcome this, fabric filters or bag filters are employed. But fabric filters arc unsuitable for wet or sticky particles, extreme corrosive conditions, and high gas temperatures.
Gaseous pollutants:
These can be removed by the following three methods.
(a) Wet systems:
These are used as washing towers in which alkali fluid circulate continuously. This liquid reacts with SO2to produce a precipitate.
(b) Dry systems:
Here the gas pollutants are allowed to react with an absorbent under a dry phase. Dolomite, lime (CaO) and limestone (CaOH) are placed in the way of the flowing gas (SO2). The process is not very expensive and does not involve any spray of water. Water in contact with SO2 produces corrosive H2SO4.
(c) Wet dry systems:
Here water in the absorbent reacts with the acid components. This offers an alternative to traditional wet process used for desulphurisation of fuel gases from coal-fired boilers. The absorbent calcium hydroxide is spread into the hot gas stream in the form of small droplets. Calcium reacts with SO2 and the hot gases cause the water to evaporate simultaneously.
The end product is a dry power containing mostly fly ash and salts. Charcoal can also be used as an absorbent. Other absorbers can also be used to pick up alcohols and benzenes. This method is very effective in dry cleaning plants, printing shops, and paint factories, food processing plants, breweries and pharmaceutical industries. Combustion of gases can also be used for petroleum industries etc.
Control through law:
Like motor vehicles, standards are to be enforced by appropriate Acts for industries also. There are other conditions that could be enforced by law.