Suspension Polymerization
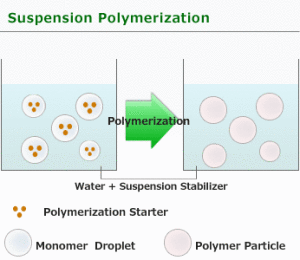
Suspension polymerization is used only in free radical type processes. The monomer is mechanically dispersed in a media, usually water. There are cases where an organic media is used in which neither the polymer nor the monomer are soluble in the organic media.
- styrene- a liquid
- vinyl chloride- a gas at room temperature
For suspension polymerization, there are two phases, water and organic, and the starting point may be 10 parts of the former, and 1 part of the latter. The initiator used can be water soluble or organic soluble [benzoyl peroxide, AIBN, or (NH4)2(SxO4)y.] Usually the initiator is organic soluble.

when using gases, pressure and containment concerns exist
- There are two separate phases throughout the whole process.
- The droplets must be kept far apart.This requires agitation: consistent, efficient, and controlled. A suspending agent can be used. Poly(vinyl alcohol) dissolved in the aqueous phase is a typical suspending agent.
- The rate of suspension polymerization is similar to the rate of bulk polymerization, but the heat transfer is much better. For suspension polymerization, initiation, propogation, and termination take place inside the droplet.
- examples include the polymerization of methyl methacrylate, and vinyl chloride.
- The solution polymerization of butadiene, isobutylene and isoprene require a pressure system.
- the media to monomer ratio is 10:1. Is this wt/vol, wt/wt, or vol/vol?? In an industrial setting it may be easier to combine chemical feedstocks on a per volume basis. Ask Dr. Stoffer.
- Particle size is affect by the following four factors:
- stirring rate
- ratio of reactants
- suspension agent
- temperature
- The plant operator must control temperature, and the particle size (of the growing polymer mass in the bubble.) If the particle size gets to large, the particle will absorb too much heat. This probably relates to the idea that as you increase the volume of a sphere, the ratio of surface area to volume decreases, and this ratio relates to heat transfer. Particle size may be 0.01 to 0.5 cm, or as low as 1 micron.
A suspension agent is a material that gives a surface activation that keeps droplets from become larger (droplets coming together to form larger droplets is called coalescence.)
I think the term coalescence is also used for when the polymer molecules in a solution come together as the solvent evaporates, an example of which is the film formation that occurs for a paint system.
Suspension polymerization is similar to bulk polyerization, and it could be considered “bulk polymerization within a droplet.” The speed at which the reaction takes place for a given temperature is the same, and just as for bulk polymerization, the kinetics or rates are proportional to monomer concentration.
There is efficient heat transfer with speed or bulk process.
Recovery of product by mechanical separation (i.e, filtration, washing) is a lot cheaper than thermo distillation. (this sentence sort of doesn’t make sense.)
The properties of the polymer are similar to those of the same polymer made by a bulk polymerization.
Limitations of suspension polymerization–
- It only applies to free radical process.
- Ionic catalysts don’t work because they compete with water.
- Agitation is critical because as the viscosity within the bead rises, the reaction rate increases suddenly (
Tromsdoiff effect.) This leads to a surge in heat generation which does not usually occur in solution or emulsion polymerization

