Planck theory
Max Planck suggested that the energy of light is proportional to its frequency, also showing that light exists in discrete quanta of energy
Planck’s law describes the electromagnetic radiation emitted by ablack body in thermal equilibrium at a definite temperature. The law is named after Max Planck, who originally proposed it in 1900. It is a pioneering result of modern physics and quantum theory.
The spectral radiance of a body, 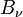 , describes the amount of energy it gives off as radiation of different frequencies. It is measured in terms of the power emitted per unit area of the body, per unit solid angle that the radiation is measured over, per unit frequency. Planck theory showed that the spectral radiance of a body at absolute temperature T is given by
, describes the amount of energy it gives off as radiation of different frequencies. It is measured in terms of the power emitted per unit area of the body, per unit solid angle that the radiation is measured over, per unit frequency. Planck theory showed that the spectral radiance of a body at absolute temperature T is given by
where kB the Boltzmann constant, h the Planck constant, and c thespeed of light in the medium, whether material or vacuum.[1][2][3] The spectral radiance can also be measured per unit wavelength instead of per unit frequency. In this case, it is given by
 .
.
The SI units are W·sr−1·m−2·Hz−1 of Bν and W·sr−1·m−3 for Bλ. The law may also be expressed in other terms, such as of the number of photons emitted at a certain wavelength, or of the energy density in a volume of radiation.
In the limit of low frequencies (i.e. long wavelengths), Planck’s law tends to the Rayleigh–Jeans law, while in the limit of high frequencies (i.e. small wavelengths) it tends to the Wien approximation.