Photosensitization
Photosensitization is a reaction to light that is mediated by a light-absorbing molecule, which is not the ultimate target. Photosensitization can involve reactions within living cells or tissues, or they can occur in pure chemical systems. In photobiology, we are concerned with the reactions in living systems.
Recall that the First Law of Photochemistry states that light (photons) must be absorbed to have an effect. In some cases, the molecule that absorbs a photon is altered chemically, but does not change any other molecule in the system. In other cases, the molecule that absorbs the photon ultimately alters another molecule in the system. In the latter process (photosensitization), the molecule that absorbs the photon is called the photosensitizer (or simply sensitizer), and the altered molecule is the acceptor or substrate. Both the photosensitizer and light are required for photosensitization.
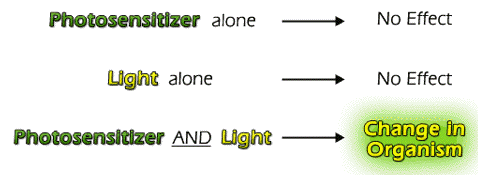
Photosensitizers are not usually consumed during photosensitization reactions. They return to their original state once the photosensitization reaction is complete. Photosensitizers can be photobleached, a chemical reaction following the absorption of light that produces a different molecular form, which does not absorb light, but this process typically competes with the photosensitization reaction.
Photosensitizers can be endogenous in living systems, or they can be taken up from exogenous sources. Endogenous photosensitizers include molecules such as porphyrins, bilirubin or chlorophyll, although in their normal cellular environment their potential photosensitizing effects are not apparent, either because their concentrations are too low, or because the molecules are sequestered in complexes that inhibit photosensitization reactions. Exogenous photosensitizers are numerous and include many varieties of dyes and biomolecules; some are natural products, e.g., from plants, and many others have been produced synthetically for medical, agricultural, or other uses. A few examples will be discussed below.
An ingenious variant on the endogenous/exogenous theme is the administration of an inert precursor, which is converted by the organism’s metabolism into a fully functional photosensitizer. For example, many plants and animals will metabolize the precursor,
 -aminolevulinic acid, to the photosensitizer protoporphyrin. Protoporphyrin is a normal intermediate in biosynthetic pathways to produce cytochromes, hemoglobin (the oxygen-binding protein in red blood cells), and myoglobin, which binds oxygen in muscle. Under normal conditions the concentrations of free protoporphyrin are too low to produce photosensitization reactions, but if excess
-aminolevulinic acid, to the photosensitizer protoporphyrin. Protoporphyrin is a normal intermediate in biosynthetic pathways to produce cytochromes, hemoglobin (the oxygen-binding protein in red blood cells), and myoglobin, which binds oxygen in muscle. Under normal conditions the concentrations of free protoporphyrin are too low to produce photosensitization reactions, but if excess  -aminolevulinic acid is provided, or if there is an impairment of normal metabolic pathways, protoporphyrin may accumulate, and photosensitivity reactions can be produced. The latter metabolic impairment is the root cause of the class of diseases known as the porphyrias.
-aminolevulinic acid is provided, or if there is an impairment of normal metabolic pathways, protoporphyrin may accumulate, and photosensitivity reactions can be produced. The latter metabolic impairment is the root cause of the class of diseases known as the porphyrias.
How Does Photosensitization Proceed?
In photosensitized reactions, the absorbed photon excites the sensitizer (abbreviated Sen in Figure 1) to one or more energy-rich state(s) (indicated by an asterisk, Sen*). The excited Sen* undergoes internal reactions that ultimately result in the chemical alteration of the substrate. This can occur in one of two types of reactions (Figure 1).
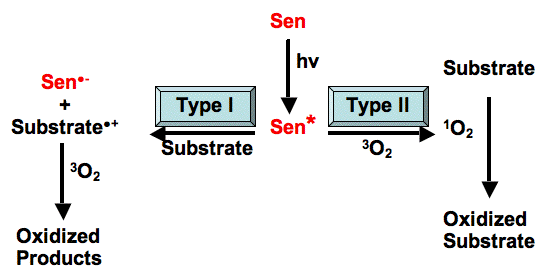
Type I vs. Type II Photosensitization Reactions.
In the Type I reaction, the excited sensitizer reacts directly with the substrate, in a one-electron transfer reaction, to produce a radical or radical ion in both the sensitizer and the substrate. Although electron transfer can proceed in either direction, usually the substrate donates an electron to the sensitizer, resulting in a substrate radical cation (Substrate.+), and a sensitizer radical anion (Sen.-). In the presence of oxygen, both of these radicals can further react to produce oxygenated products. This type of reaction can lead to a loss of the sensitizer, since it is converted to an oxidized molecule. Another possible reaction is the direct transfer of the extra electron of Sen.- to oxygen to produce the superoxide radical anion (O2.-), and regenerating the original sensitizer (Sen).
In the Type II reaction, the excited sensitizer transfers its excess energy to ground-state molecular oxygen (3O2), producing excited state singlet oxygen (1O2), and regenerating the ground-state sensitizer. Singlet oxygen then reacts with the substrate to generate oxidized products. The photosensitizer is not consumed during this type of photosensitized reaction.

