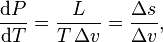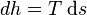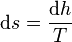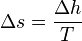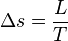Clausius-Clapeyron Equation
The Clausius–Clapeyron relation, named after Rudolf Clausius and Benoît Paul Émile Clapeyron, is a way of characterizing a discontinuous phase transition between two phases of matter of a single constituent. On a pressure–temperature (P–T) diagram, the line separating the two phases is known as the coexistence curve. The Clausius–Clapeyron relation gives the slope of the tangents to this curve. Mathematically,
where 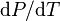 is the slope of the tangent to the coexistence curve at any point,
is the slope of the tangent to the coexistence curve at any point,  is the specific latent heat,
is the specific latent heat,  is the temperature,
is the temperature, 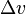 is the specific volume change of the phase transition and
is the specific volume change of the phase transition and 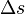 is the entropy change of the phase transition.
is the entropy change of the phase transition.
Derivation from state postulate
Using the state postulate, take the specific entropy  for a homogeneous substance to be a function of specific volume
for a homogeneous substance to be a function of specific volume  and temperature
and temperature  .
.
The Clausius–Clapeyron relation characterizes behavior of a closed system during a phase change, during which temperature and pressureare constant by definition. Therefore,[3]:508
Using the appropriate Maxwell relation gives
where 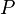 is the pressure. Since pressure and temperature are constant, by definition the derivative of pressure with respect to temperature does not change.[4][5]:57, 62 & 671 Therefore the partial derivative of specific entropy may be changed into atotal derivative
is the pressure. Since pressure and temperature are constant, by definition the derivative of pressure with respect to temperature does not change.[4][5]:57, 62 & 671 Therefore the partial derivative of specific entropy may be changed into atotal derivative
and the total derivative of pressure with respect to temperature may be factored out when integrating from an initial phase  to a final phase
to a final phase 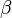 , to obtain
, to obtain
where 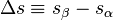 and
and 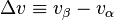 are respectively the change in specific entropy and specific volume. Given that a phase change is an internally reversible process, and that our system is closed, the first law of thermodynamics holds
are respectively the change in specific entropy and specific volume. Given that a phase change is an internally reversible process, and that our system is closed, the first law of thermodynamics holds
where 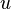 is the internal energy of the system. Given constant pressure and temperature (during a phase change) and the definition of specific enthalpy
is the internal energy of the system. Given constant pressure and temperature (during a phase change) and the definition of specific enthalpy 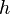 , we obtain
, we obtain
Given constant pressure and temperature (during a phase change), we obtain
Substituting the definition of specific latent heat 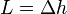 gives
gives
Substituting this result into the pressure derivative given above (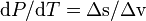 ), we obtain
), we obtain
This result (also known as the Clapeyron equation) equates the slope of the tangent to the coexistence curve 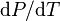 , at any given point on the curve, to the function
, at any given point on the curve, to the function 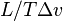 of the specific latent heat
of the specific latent heat  , the temperature
, the temperature  , and the change in specific volume
, and the change in specific volume 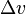 .
.