Chemiluminescence
Chemiluminescence is the emission of light (luminescence), as the result of a chemical reaction. There may also be limited emission of heat. Given reactants A and B, with an excited intermediate◊,
- [A] + [B] → [◊] → [Products] + light
For example, if [A] is luminol and [B] is hydrogen peroxide in the presence of a suitable catalyst we have:
- luminol + hydrogen peroxide → 3-APA[◊] → 3-APA + light
where:
- 3-APA is 3-aminophthalate
- 3-APA[◊] is the vibronic excited state fluorescing as it decays to a lower energy level.
The decay of this excited state[◊] to a lower energy level causes light emission. In theory, one photon of light should be given off for each molecule of reactant. This is equivalent to Avogadro’s number of photons per mole of reactant. In actual practice, non-enzymatic reactions seldom exceed 1% QC, quantum efficiency.
In a chemical reaction, reactants collide to form a transition state, the enthalpic maximum in a reaction coordinate diagram, which proceeds to the product. Normally, reactants form products of lesser chemical energy. The difference in energy between reactants and products, represented as 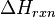 , is turned into heat, physically realized as excitations in the vibrational state of the normal modes of the product. Since vibrational energy is generally much greater than the thermal agitation, it rapidly disperses in the solvent through molecular rotation. This is how exothermic reactions make their solutions hotter. In a chemiluminescent reaction, the direct product of a reaction is an excited electronic state, which then decays into an electronic ground state through either fluorescence or phosphorescence, depending partly on the spin state of the electronic excited state formed.
, is turned into heat, physically realized as excitations in the vibrational state of the normal modes of the product. Since vibrational energy is generally much greater than the thermal agitation, it rapidly disperses in the solvent through molecular rotation. This is how exothermic reactions make their solutions hotter. In a chemiluminescent reaction, the direct product of a reaction is an excited electronic state, which then decays into an electronic ground state through either fluorescence or phosphorescence, depending partly on the spin state of the electronic excited state formed.
Chemiluminescence differs from fluorescence in that the electronic excited state is derived from the product of a chemical reaction rather than the more typical way of creating electronic excited states, namely absorption. It is the antithesis of a photochemical reaction, in which light is used to drive an endothermic chemical reaction. Here, light is generated from a chemically exothermic reaction.
A standard example of chemiluminescence in the laboratory setting is the luminol test. Here, blood is indicated by luminescence upon contact with iron in hemoglobin. When chemiluminescence takes place in living organisms, the phenomenon is called bioluminescence. A light stick emits light by chemiluminescence.

