Brass
Brass is an alloy made of copper and zinc; the proportions of zinc and copper can be varied to create a range of brasses with varying properties. It is a substitutional alloy: atoms of the two constituents may replace each other within the same crystal structure.
By comparison, bronze is principally an alloy of copper and tin. However, the common term “bronze” may also include arsenic, phosphorus, aluminium,manganese, and silicon. The term is also applied to a variety of brasses, and the distinction is largely historical. Modern practice in museums and archaeology increasingly avoids both terms for historical objects in favour of the all-embracing “copper alloy”.
Brass is used for decoration for its bright gold-like appearance; for applications where low friction is required such as locks, gears, bearings, doorknobs, ammunition casings and valves; for plumbing and electrical applications; and extensively in brass musical instruments such as horns and bells where a combination of high workability (historically with hand tools) and durability is desired. It is also used in zippers. Brass is often used in situations in which it is important that sparks not be struck, such as in fittings and tools around explosive gases.
Properties
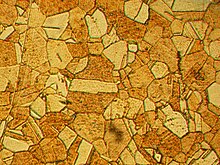
Microstructure of rolled andannealed brass (400X magnification)
The malleability and traditionally attributed acoustic properties of brass have made it the metal of choice for musical instruments such as the trombone, tuba, trumpet,cornet, baritone horn, euphonium, tenor horn, and French horn which are collectively known as brass instruments. Even though the saxophone is classified as a woodwind instrument and the harmonica is a free reed aerophone, both are also often made from brass. In organ pipes of the reed family, brass strips (called tongues) are used as the reeds, which beat against the shallot (or beat “through” the shallot in the case of a “free” reed). Although not part of the brass section, snare drums are also sometimes made of brass.
Brass has higher malleability than bronze or zinc. The relatively low melting point of brass (900 to 940 °C, 1652 to 1724 °F, depending on composition) and its flow characteristics make it a relatively easy material to cast. By varying the proportions of copper and zinc, the properties of the brass can be changed, allowing hard and soft brasses. The density of brass is approximately .303 lb/cubic inch, 8.4 to 8.73 grams per cubic centimetre.
Today almost 90% of all brass alloys are recycled. Because brass is not ferromagnetic, it can be separated from ferrous scrap by passing the scrap near a powerful magnet. Brass scrap is collected and transported to the foundry where it is melted and recast into billets. Billets are heated and extruded into the desired form and size.
Aluminium makes brass stronger and more corrosion resistant. Aluminium also causes a highly beneficial hard layer ofaluminium oxide (Al2O3) to be formed on the surface that is thin, transparent and self-healing. Tin has a similar effect and finds its use especially in seawater applications (naval brasses). Combinations of iron, aluminium, silicon and manganese make brass wear and tear resistant.

