Alloys
|
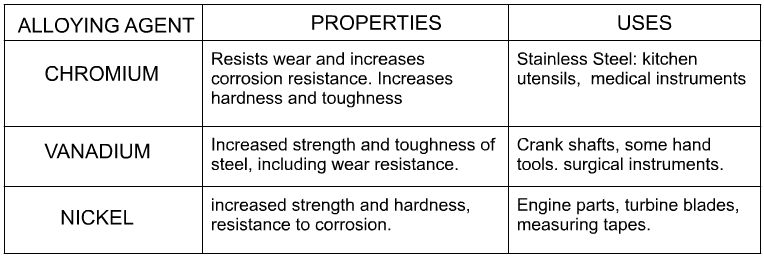
An alloy is a metal (parent metal) combined with other substances (alloying agents), resulting in superior properties such as; strength, hardness, durability, ductility, tensile strength and toughness. The parent metal is the majority of the alloy. For example, mild steel is 0.1 – 0.3% Carbon and 99.9 – 99.7% Iron. Alloys are sometimes described as a mixture of two or more metals. However, this is misleading, as often alloys are composed of just one metal, as well as other non-metal elements. Cast iron is an example, as it is a combination of iron (metal) and carbon (non-metal). Metal alloys have specific enhanced properties compared to their ‘parent’ metals. |