Absorption
Absorption of electromagnetic radiation is the way in which the energy of a photon is taken up by matter, typically the electrons of an atom. Thus, the electromagnetic energy is transformed into internal energy of the absorber, for example thermal energy. The reduction in intensity of a light wave propagating through a medium by absorption of a part of its photons is often called attenuation. Usually, the absorption of waves does not depend on their intensity (linear absorption), although in certain conditions (usually, in optics), the medium changes its transparency dependently on the intensity of waves going through, and saturable absorption (or nonlinear absorption) occurs.
Applications
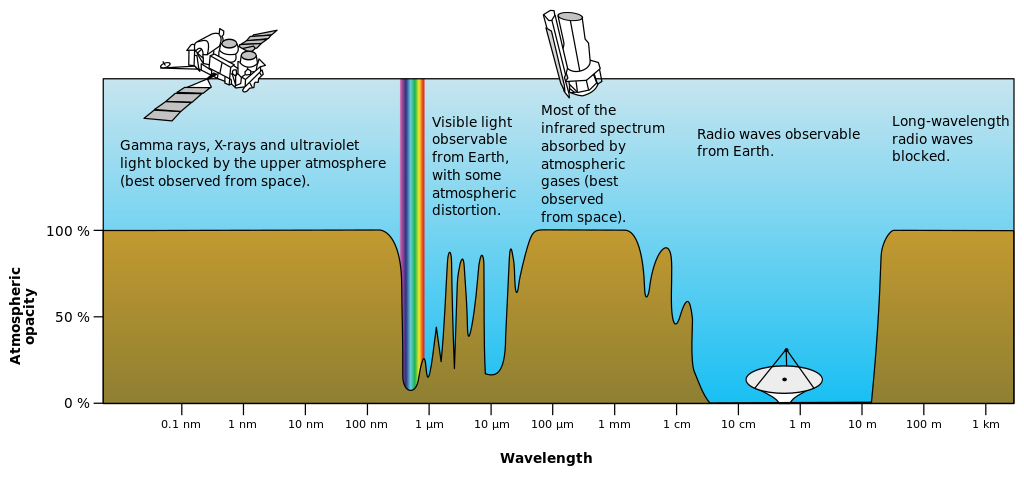
Rough plot of Earth’s atmospheric transmittance (or opacity) to various wavelengths of electromagnetic radiation, including visible light.
Understanding and measuring the absorption of electromagnetic radiation has a variety of applications. Here are a few examples:
- In meteorology and climatology, global and local temperatures depend in part on the absorption of radiation by atmospheric gases (such as in the greenhouse effect) and land and ocean surfaces
- In medicine, X-rays are absorbed to different extents by different tissues (bone in particular), which is the basis for X-ray imaging. For example, see computation of radio wave attenuation in the atmosphere used in satellite link design.
- In chemistry and materials science, because different materials and molecules will absorb radiation to different extents at different frequencies, which allows for material identification.
- In optics, sunglasses, colored filters, dyes, and other such materials are designed specifically with respect to which visible wavelengths they absorb, and in what proportions.
- In biology, photosynthetic organisms require that light of the appropriate wavelengths be absorbed within the active area of chloroplasts, so that the light energy can be converted into chemical energy within sugars and other molecules.

