Van ‘t Hoff isotherm
The Gibbs free energy can change with the change of the temperature and pressure of the thermodynamic system. TheVan ‘t Hoff isotherm can be used to determine the Gibbs free energy for non-standard state reactions at a constant temperature:
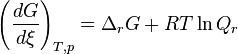
犀利士
where  is the Gibbs free energy for the reaction, and
is the Gibbs free energy for the reaction, and 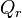 is the reaction quotient. When a reaction is at equilibrium,
is the reaction quotient. When a reaction is at equilibrium, 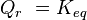 . The Van ‘t Hoff isotherm can help estimate the equilibrium reaction shift. When
. The Van ‘t Hoff isotherm can help estimate the equilibrium reaction shift. When  , the reaction moves in the forward direction. When
, the reaction moves in the forward direction. When  , the reaction moves in the backwards directions. See Chemical equilibrium.
, the reaction moves in the backwards directions. See Chemical equilibrium.
Van ‘t Hoff plot
For a reversible reaction, the equilibrium constant can be measured at a variety of temperatures. This data can be plotted on a graph with 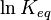 on the Y-axis and
on the Y-axis and  on the X-axis. The data should have a linear relationship, the equation for which can be found by fitting the data using the linear form of the Van ‘t Hoff equation
on the X-axis. The data should have a linear relationship, the equation for which can be found by fitting the data using the linear form of the Van ‘t Hoff equation
This graph is called the Van ‘t Hoff plot and is widely used to estimate the enthalpy and entropy of a chemical reaction. From this plot,  is the slope and
is the slope and  is the intercept of the linear fit.
is the intercept of the linear fit.
By measuring the equilibrium constant, Keq, at different temperatures, the Van ‘t Hoff plot can be used to assess a reaction when temperature changes. Knowing the slope and intercept from the Van ‘t Hoff plot, the enthalpy and entropy of a reaction can be easily obtained using
![]()
![]()
The Van ‘t Hoff plot can be used to quickly determine the enthalpy of a chemical reaction both qualitatively and quantitatively. Change in enthalpy can be positive or negative, leading to two major forms of the Van ‘t Hoff plot.
Endothermic reactions

Endothermic Reaction Van ‘t Hoff Plot
For an endothermic reaction, heat is absorbed, making the net enthalpy change positive. Thus, according to the definition of the slope:
![]()
for an endothermic reaction,
 and R is the gas constant
and R is the gas constant
So
![]()
Thus, for an endothermic reaction, the Van ‘t Hoff plot should always have a negative slope.
Exothermic reactions
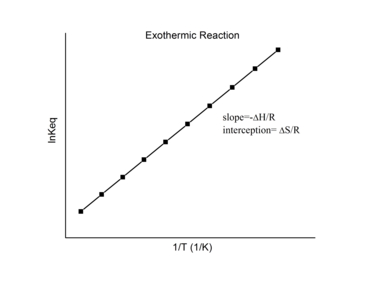
Exothermic Reaction Van ‘t Hoff Plot
For an exothermic reaction, heat is released, making the net enthalpy change negative. Thus, according to the definition of the slope:
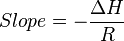
from an exothermic reaction,
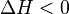 and R is the gas constant
and R is the gas constant
So
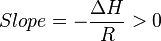
Thus, for an exothermic reaction, the Van ‘t Hoff plot should always have a positive slope.

